
Each oxide has a charge of 2-, which means that we can get 6 negative charges by having 3 oxides. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration Kr4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. Elements position on the table tells you their valence. The third major category of elements arises when the distinguishing electron occupies an f subshell.

All of the elements of this family can form four bonds, the most of any family. Each aluminium has a charge of 3+, which means that we can get 6 positive charges by having 2 aluminiums. You can use a Periodic Table to find the charge that an element is likely to have as an ion. They usually form compounds as cations with a +4 charge. On the Periodic Table metals (found on the left of the table) will be. This means that we can balance the charge of this ionic compound by having 6 positive charges and 6 negative charges. To find the ionic charge of an element youll need to consult your Periodic Table. The first number that 3 and 2 go into is 6 (the LCM is 6). Indulge your curiosity with our complete periodic table of elements with masses, charges, chemical types, and everything else you can think of Our mobile.

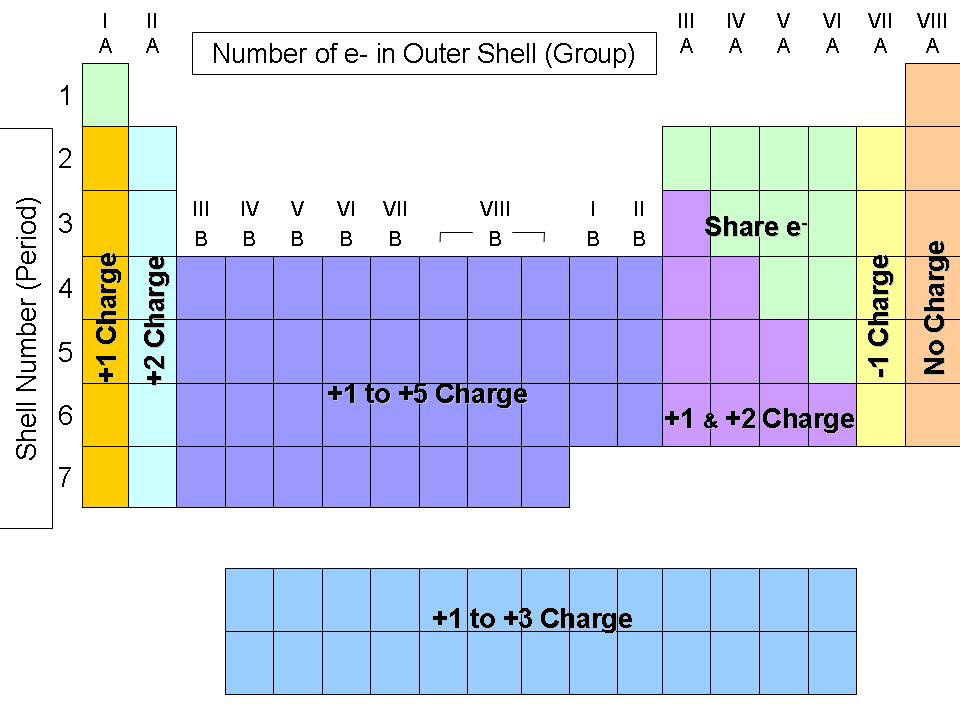
The balancing for aluminium oxide is slightly different because the positive and negative charges do not go into each other the 3 positive charge from aluminium and the 2 negative charge from oxygen do not go into each other. The overall charge for an ionic compound must be 0, which means that the charges from aluminium and oxygen must balance out. These printable periodic tables list the element charges right on them, so you don’t have to try to figure them out based on the location of the element group. Oxygen is in group 6 in the periodic table, which means that it will gain 2 electrons resulting in it having a 2 negative charge (O 2-). Aluminium is a metal and is in group 3 in the periodic table, which means that it will lose 3 electrons resulting in it having a 3 positive charge (Al 3+). You can find a periodic table online or in a chemistry book. However, the Periodic table generally displays only the symbol of the element and not its entire name. Other elemental information includes atomic weight and atomic number. The periodic table of elements is widely used in the field of Chemistry to look up chemical elements as they are arranged in a manner that displays periodic trends in the chemical properties of the elements. The atomic number is how many protons the element’s atom possesses. The elements are ordered by their atomic numbers, which increase as you move across and down the periodic table. Read the periodic table from top left to bottom right. A trend is to find the charge by looking at the Group on the Periodic. Be especially careful with four (IV in Roman numerals) and six (VI in Roman numerals). Recognizing the Structure of the Periodic Table. When writing formulas for ions and ionic compounds we often need to find the ionic charge. In many cases, the elements position on the periodic table will help you determine the kind of ion formed (anion or cation) and the size of the ionic charge.
#ELEMENT TABLE WITH CHARGES HOW TO#
For a reminder about how to write Roman numerals, see Table 6.2. 1: The periodic table showing elements that form ions with variable charges. It is color-coded and assigns each element a unique 1 or 2-letter abbreviation. the iron (III) ion has a chemical formula of Fe 3+. This periodic table with charges is a useful way to keep track of the most common oxidation numbers for each element. What is the empirical formula of aluminium oxide?Īluminium oxide is made out of aluminium and oxygen atoms. The periodic table is a chart that organizes elements by their atomic structure.


 0 kommentar(er)
0 kommentar(er)
